JSR Succeeds in Developing Biomimetic Smart Plastic Materials for Precise Recognition of Antibodies
Tokyo, Japan - August 21, 2024 - JSR Corporation (Headquarters: Minato-ku, Tokyo CEO: Eric Johnson), succeeded in developing a smart plastic material that can precisely recognize antibodies, much like biomolecule protein A (hereafter “PA”), by using a bridging surface modification technology with our original epoxide linking reaction1. As a low-cost alternative to PA created with simple surface modification technology, this material is expected to be used in a wide variety of applications such as antibody purification and diagnostic biosensors.
An article on this research was published in the volume 11, issue 23, 2024 of Advanced Materials Interfaces by Wiley-VCH2 and selected for the front cover of this issue (Figure A, right) demonstrating the novelty and potentiality of this research. Regarding this case, the researcher has commented, "The trial and error of applying our original reaction to the materials managed in daily work led to the creation of novel technology from scratch."
The findings will also be presented at the 105th Annual Meeting of the Chemical Society of Japan to be held in March 2025.Antibody-binding proteins, such as PA, specifically bind strongly to an antibody and can release the bound antibody in response to changes in pH of the buffers (buffered aqueous solution). Utilizing such unique properties, the molecule is applied to purification of pharmaceutical antibodies and biosensors. While the PA resin market size was said to be approximately USD 1 billion in FY2022, and is expected to grow further, there are still issues related to biomolecules, such as manufacturing costs, and contamination, and thus the development of non-biomolecular alternative materials is anticipated.
In this study, JSR discovered that porous microspheres for column purification modified with the molecular-level bridging structure using our original epoxides linking reaction (Figure B) exhibit IgG binding selectivity, buffer responsiveness, and Fc site-specific recognition, which is very similar to PA for a type of antibody, immunoglobulin G (IgG) (Figure C). Using these characteristics, we have successfully demonstrated in easily separating mixtures of IgG and model proteins solely by buffer exchange.
To determine the origin of these characteristics, a series of microspheres with analogical surface-modified structures was investigated. Unlike non-bridging analog structures, only a series of the bridged structures bound to IgG. This indicated that the bridging structures play a predominant role in IgG recognition. It can be said that the newly developed bridging surface modification is a new technology to highly functionalize plastic materials while being a simple and low-cost process.In future research and development, we will explore new initiatives, such as collaborating with external research institutes and promoting the development of materials that can contribute to society.
- 1. Our original epoxide linking reaction:
- 2. Published paper: “A Thioether-bridging Surface Modification of Polymeric Microspheres Offers Nonbiological Protein A-mimetic Affinity for IgG“

Figure A. Series of research results related to this material: back cover of Chemical Communications published by the Royal Society of Chemistry (left); cover of Advanced Materials Interface published by Wiley-VCH (right).
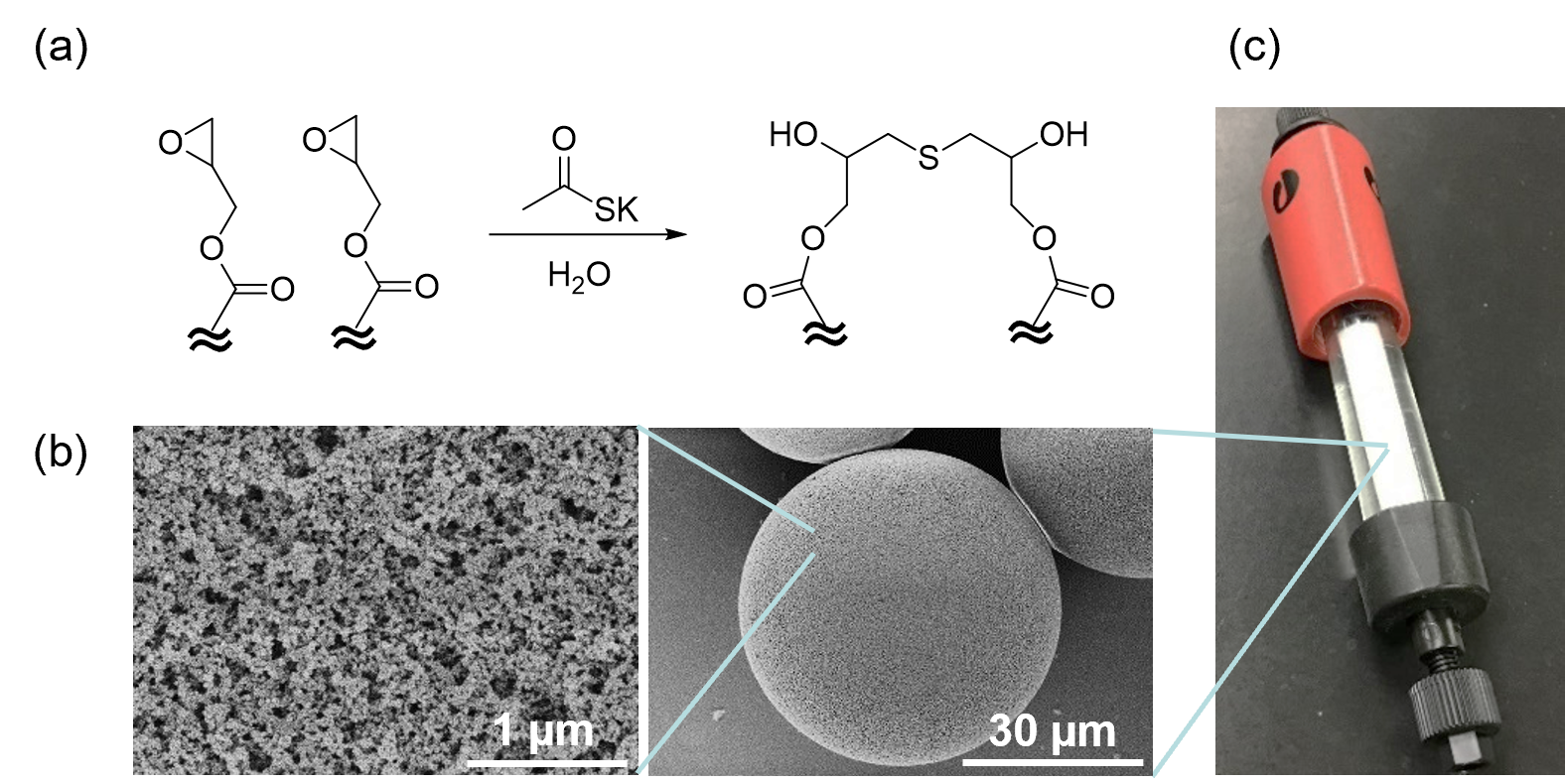
Figure B. Bridge-modified-porous polymeric microspheres as chromatographic column support.
(a) Thioether-bridging structure on microspheres formed by epoxide linking reaction.
(b) Scanning electron microscopy images of surface-modified porous microspheres.
(c) Microspheres-packed column.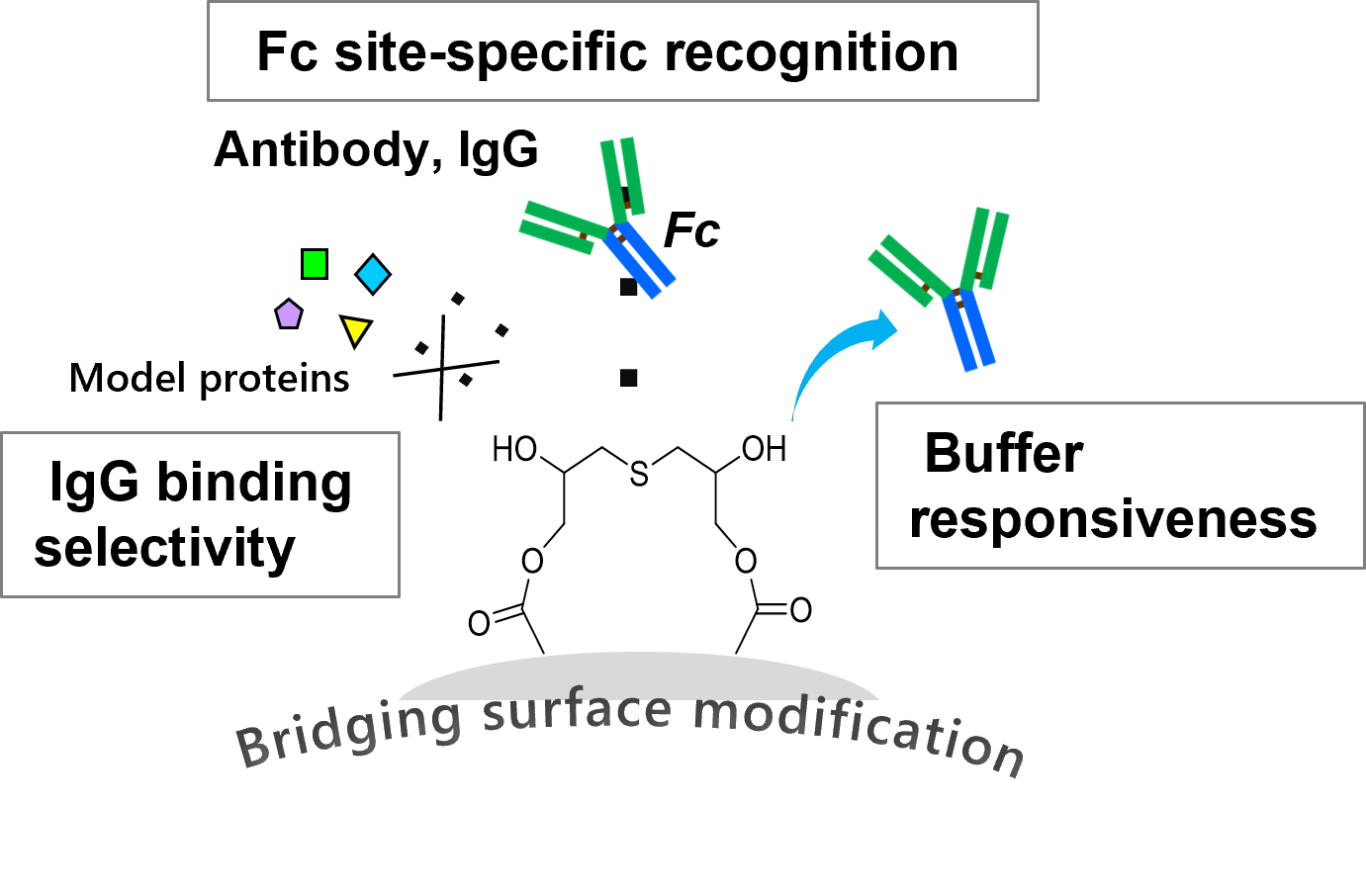
Figure C. Conceptual image of IgG recognition performance of the developed microspheres.
*IgG binding selectivity: A binding evaluation to IgG and several model proteins revealed that the microspheres bind selectively to IgG in neutral buffer (pH 7.5).
*Buffer responsiveness: The bound IgG was released rapidly solely by buffer exchange from neutral-pH buffer to acidic buffer (pH 3.2).
*Fc site-specific recognition: IgG has a structure looking like the letter "Y". The microspheres selectively binds to the Fc region which is the lower half region of IgG.