Success in Highly Efficient Linking of Two Epoxides under Nonstoichiometric Condition
JSR Corporation (Headquarters: Minato-ku, Tokyo CEO: Eric Johnson), in collaboration with the National Institute for Materials Science (NIMS), has succeeded in the highly efficient linking of two epoxides under nonstoichiometric*1 conditions.
An article on this research was published in “Chemical Communications” published by the Royal Society of Chemistry (RSC) *2 on Jan. 25th, 2022 and was selected for the back cover of this issue (Figure A) demonstrating the novelty and potentiality of this research.
Linking of epoxides using a cross-linking agent such as diamine and dithiol is an indispensable technique for many kinds of production, especially the manufacture of epoxy resin, which is widely used for many applications such as adhesives and coatings. According to the latest report, the global annual demand for epoxy resin has been estimated at more than 3.5 million tons.
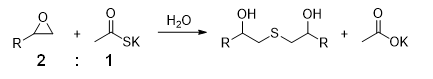
Though seemingly exhaustive studies on the basic techniques of linking methods had been conducted over the past several decades, A JSR researcher discovered that two epoxides can be efficiently linked using potassium thioacetate (AcSK) in water even at imbalanced stoichiometric ratios (Figure B).
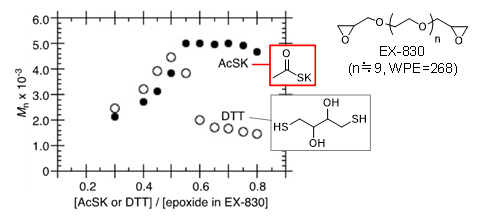
Unlike reactions using a conventional cross-linking agent, it is possible to readily produce the desired compounds by roughly controlling the ratio of raw materials. Model reactions using twice the amount of cross-linking agent than required showed that the yield of the reaction using AcSK reached 99% while one using a conventional cross-linking agent (DTT: dithiothreitol) was approximately 33%. By introducing bifunctional epoxides we achieved a nonstoichiometric polymerization which gave the polymers a nearly constant molecular weight under nonstoichiometric conditions (Figure C). This is the first example of nonstoichiometric polymerization of epoxides. We also demonstrated its effectiveness in the formation of hydrogels and self-standing films.
Given the versatility of the reagents, epoxide and AcSK, we expect that this unique reaction could lead to new industrial applications of epoxides, for not only existing markets, but also fields such as electronics and life sciences.
JSR will give a presentation on this research at the 71st Annual Meeting of the Society of Polymer Science, Japan (SPSJ) in May 2022.
* 1 Stoichiometry
The quantitative relationship of chemical species (molecules) in a chemical reaction. In linking reaction shown here, the stoichiometric ratio is 2:1 (mol/mol) because two epoxides react with one potassium thioacetate. Non-stoichiometric condition refers to reaction condition out of the ratio.
*2. Published papers
Efficient linking of two epoxides using potential thioacetate in water and its use in polymerization
Takanori Kishida *, Tomio Shimada and Kazunori Sugiyasu *, Chem. Commun., 2022, 58, 1108-1110.
A. Outside Back Cover, 2022 58, 1108-1110. issue of Chemical Communications, published by the Royal Society of Chemistry

- B. Chemical Reaction Scheme of Two Molecules of Epoxide and One Molecule of Potassium Thioacetate
-
- C. Relationship between Molecular Weight (Mn) of Polymers Obtained from Bifunctionalepoxide EX -830 using cross-linking agent and Stoichiometric Ratio